Baseline Harmonize
Save Weeks on SDTM Creation Without Sacrificing Quality
Description
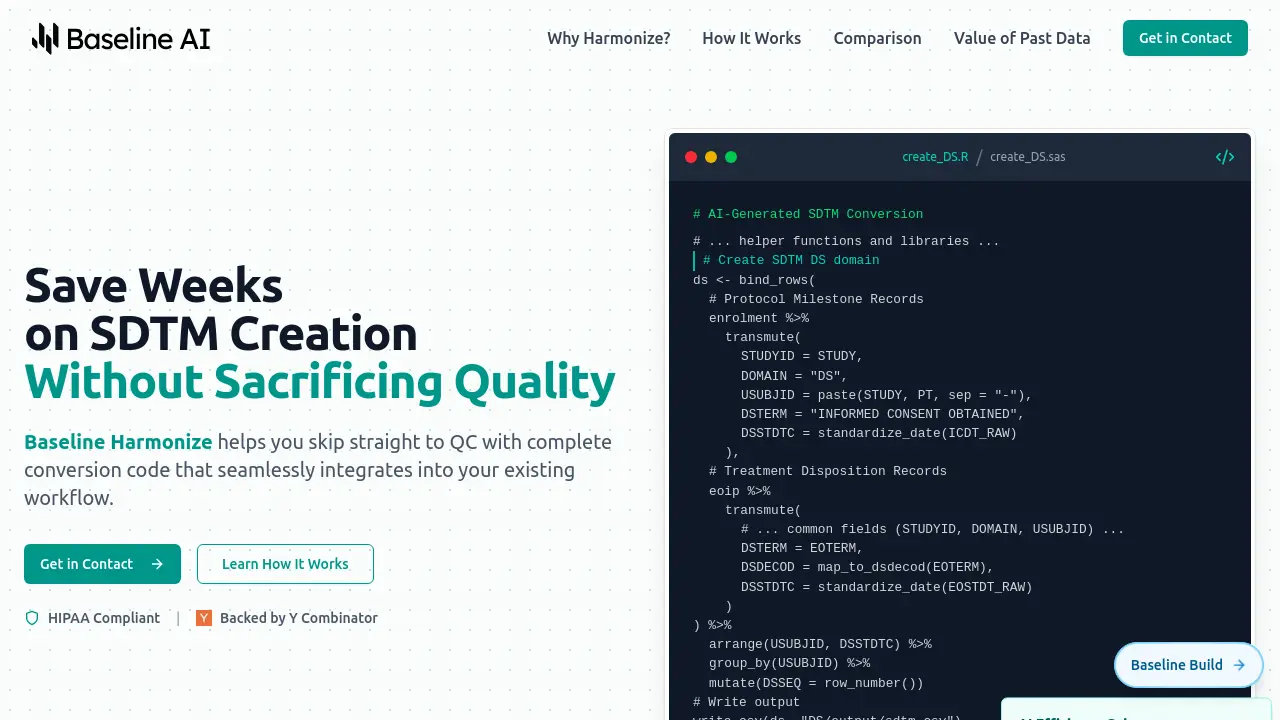
Baseline Harmonize assists clinical programming teams by automating the generation of Study Data Tabulation Model (SDTM) conversion code. Leveraging artificial intelligence trained on the latest CDISC guidelines, including SDTMIG v3.4 and Controlled Terminology, the tool transforms source clinical trial datasets into standardized formats. It generates both mapping specifications and ready-to-use conversion code in familiar languages like SAS and R, designed to integrate seamlessly into existing programming workflows.
This AI tool significantly reduces the time typically spent on initial spec writing and manual coding, potentially cutting a multi-week process down by several weeks. Beyond streamlining current study conversions, Baseline Harmonize also enables the harmonization of disparate historical clinical trial data into a unified, FAIR (Findable, Accessible, Interoperable, Reusable) SDTM format. This capability unlocks valuable insights from past studies, supports cross-trial analysis, and creates AI-ready datasets, ultimately maximizing the return on investment in clinical data.
Key Features
- AI-Generated SDTM Conversion: Automatically creates mapping specifications and conversion code (SAS/R).
- Workflow Integration: Produces standard, readable code that fits into existing clinical programming environments.
- Accelerated Timeline: Reduces initial SDTM mapping time by up to 93%, shortening the overall process.
- CDISC Compliance: Built-in knowledge of SDTMIG v3.4 and Controlled Terminology ensures regulatory alignment.
- Maintains Control: Programmers review, edit, and approve AI-generated code.
- Historical Data Harmonization: Unifies past clinical trial data into a single, standardized SDTM format.
- FAIR Data Enablement: Helps make clinical data Findable, Accessible, Interoperable, and Reusable.
Use Cases
- Accelerating SDTM dataset creation for regulatory submissions.
- Reducing manual coding effort and time for clinical programmers.
- Standardizing and harmonizing historical clinical trial data.
- Creating analysis-ready datasets for cross-study insights.
- Preparing clean, structured data for AI/ML model training in clinical research.
- Improving adherence to FAIR data principles within organizations.
You Might Also Like
Profiler
FreeCrafts Insightful Profiles by Analyzing Social Feeds
Animaker
FreemiumThe Future of Video Making Starts Here.
Whatmore
FreemiumEffortlessly create and use highly converting videos for your Website, Ads, Marketplace listings and Socials
varys.ai
FreeTagline Placeholder
FORA
PaidCapture, preserve, and analyze Communication Intelligence to deliver actionable insights.